Isotopes of hydrogen
Hydrogen is the first element in the periodic table. It has the simplest electronic configuration 1s1. It contains one proton in the nucleus and one electron.
Explanation: By the definition of isotopes, any atom which has same proton but differ in number of neutrons. In the given element which is Hydrogen. Which has three isotopes 1H, 2H and 3H. It has the same proton which is 1 and neutrons ranges from zero to 2. 1H has no neutrons, 2H has one neuton and 3H has two neutrons.
Heavier Synthetic Isotopes. 4 H contains one proton and three neutrons in its nucleus. It is a highly unstable isotope of hydrogen. It has been synthesized in the laboratory by bombarding tritium with fast-moving deuterium nuclei. Hydrogen (1 H) has three naturally occurring isotopes, sometimes denoted 1 H, 2 H, and 3 H. The first two of these are stable, while 3 H has a half-life of 12.32 years. There are also heavier isotopes, which are all synthetic and have a half-life less than one zeptosecond (10 −21 second). Of these, 5 H is the most stable, and 7 H is the least.
Isotopes:- Atoms of the same element having same atomic number but different mass number are called isotopes.
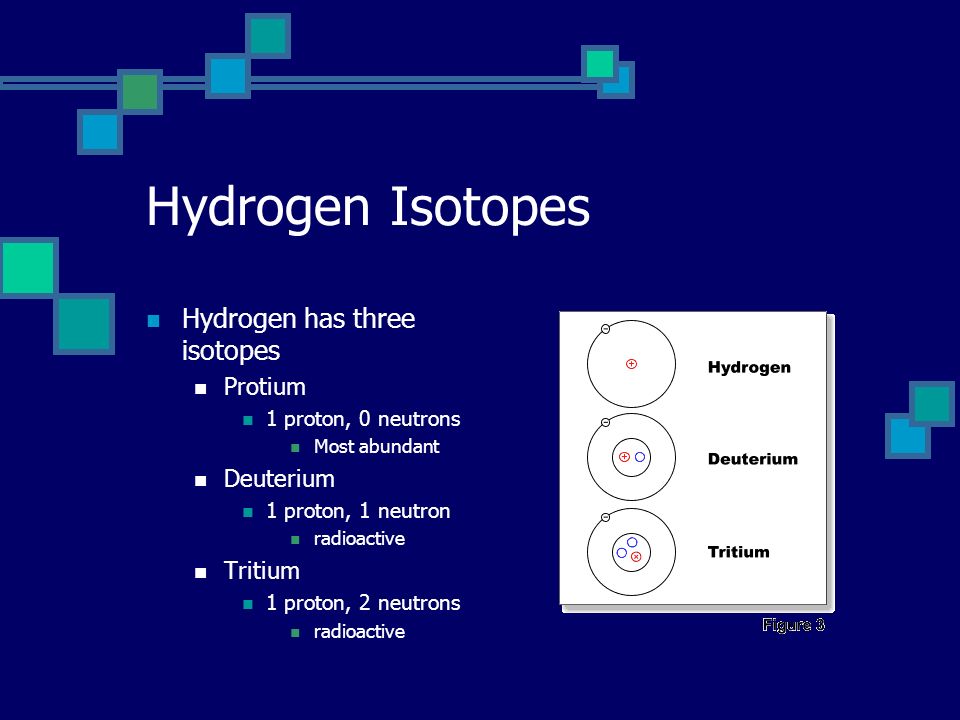
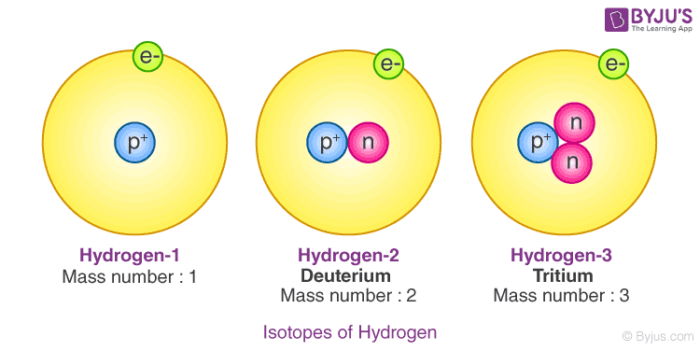
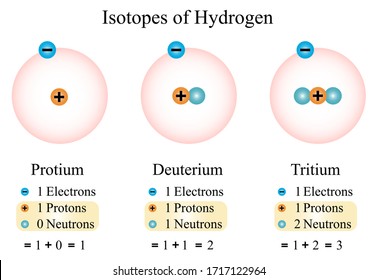
There are three isotopes for hydrogen with mass numbers 1, 2 and 3, each possessing an atomic number of one.
The structure of the three isotopes of hydrogen are
1.Protium or ordinary hydrogen: It is the common form of hydrogen. Itconsists of one proton in its nucleus and one electron revolving around it. It constitutes 99.984% of total hydrogen available in nature. Its mass number is one.
2.Deuterium or heavy hydrogen: 1H2or1D2. It occurs naturally in verysmall traces. The proportion present in naturally occurring hydrogen is in the approximate ratio: D: H~ 1:6000. It's nucleus consists of a proton and a neutron. However only a solitary electron is revolving around the nucleus. Its chemical properties are similar to those of protium but their reaction rates are different.
3.Tritium, 1H3 or 1T3: It occurs in the upper atmosphere only whereit is continuously formed by nuclear reactions induced by cosmic rays. Unlike deuterium, it is radioactive, with a half-life of ~ 12.3 years. It's nucleus consists of one proton and two neutrons.
They will have same similar chemical properties, however, their reaction rates will be different and their physical properties differ appreciably.
Isotopes of hydrogen :
S. Atomic Mass Number of Percentage
Name Symbol
No number number Pro- Neu- abundance
tons trons
1. Protium or 1H1 1 1 1 0 99.984
hydrogen
2. Deuterium 1H2 1 2 1 1 0.016
or heavy
Isotopes Of Phosphorus
hydrogen
Three Isotopes Of Hydrogen Are
3. Tritium 1H3 1 3 1 2 10-15
